
Retina
Latest News
EURETINA 2024: Genotype-phenotype correlations in a cohort of patients with genetically determined retinitis pigmentosa

EURETINA 2024: The global VOYAGER study provides real-world, scalable findings
Latest Videos

CME Content
More News

The general secretary of the European Society of Retina Specialists (EURETINA) says the future of AI is here

Editorial advisory board member Alexandra Miere, MD, PhD, says oculomics is the next big thing in eye care
Additional previous surgeries and the duration of the SO tamponade had little or no impact

Veeral Sheth, MD, MBA, FASRS, FACS, discusses outcomes from the Phase 1 HELIOS trial

New research and EURETINA highlights from the director of the "underdog" eye clinic

How retinal imaging can provide clinicians a deeper understanding of myopia

Yousif Subhi, MD, PhD, discusses his Ophthalmologica Lecture, "Things That Matter: An Evidence-Based Approach to AMD and CSC"

The CHMP will recommend SB15, biosimilar to aflibercept (Eylea), for marketing authorisation by the European Commission

Research findings from Moorfields Eye Hospital also indicate SDD and drusen differ in many factors related to stroke risk

We spoke with Andrea Govetto, MD, PhD, winner of the inaugural Ramin Tadayoni Award at EURETINA

Researchers in Portugal investigated Usher syndrome, Senior-Loker syndrome and other ciliopathies
Why having a set process for diagnostic imaging is crucial to patient outcomes

Bothnia Dystrophy generally presents during early childhood; patients have night blindness, progressive visual loss, and subsequent legal blindness.
A case series illuminates neuro-ophthalmologic manifestations, retinal vascular involvement and other pathologies
The research team used spectral-domain OCT and OCT angiography imaging to study the changes in the retinal microvasculature in patients

Dr Margaret A. Chang shares data on the port delivery system with ranibizumab, and her strategy for making the most of conferences

Spotlight on the retina specialists who make up the Eye Care Network advisory boards

Businesses in the retina care space tease new technologies and cutting-edge therapeutics

As part of the Barcelona meeting, retina specialists can recognise the awareness day early

Focus on collaboration, career development and innovation

The Ramin Tadayoni Award supports the next generation of retina researchers while paying tribute to a leader in the field

The future of the tech-forward surgical suite

A meta-analysis indicates a "significant association" between vitamin D deficiency and increased risk of RVO
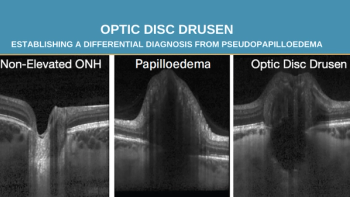
Clinical associations, imaging strategies and establishing a differential diagnosis from pseudopapilloedema

Lupin has successfully completed a clinical study of LUBT010, a ranibizumab biosimilar to Lucentis




































