
The European Medicines Agency's new standard procedure allows manufacturers to request advice from medical device expert panels
Grace Koennecke is an Editorial Intern for MJH Life Sciences.

The European Medicines Agency's new standard procedure allows manufacturers to request advice from medical device expert panels
OCU400 is the first gene therapy from the biotechnology company to move forward into Phase 3 with a broad retinitis pigmentosa (RP) indication

The lead gene therapy candidate, AXV-101, is expected to enter clinical development in mid-2025

The scientific outputs from MACUSTAR’s study could impact more than 200 million patients with AMD globally
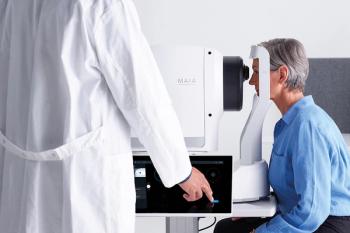
The updated device is designed to meet market diagnostic needs, including within geographic atrophy research

The company, which has European headquarters in Basel, Switzerland, plans to use the funds for a non-interventional, observational study and a first-in-human clinical trial

The ALLY Adaptive Cataract Treatment System will be the first EU ALLY commercial installation

The EMA issued a designation for the small molecule photoswitch, which targets inherited retinal diseases including retinitis pigmentosa
Published: February 3rd 2025 | Updated: March 4th 2025

Published: February 3rd 2025 | Updated: March 4th 2025

Published: January 16th 2025 | Updated: