Retina
Latest News

Internal limiting membrane staining dye ILM-Blue from DORC (Zeiss) receives NMPA approval in China
Latest Videos

CME Content
More News

The Combination OPT-302 with Aflibercept Study (COAST) evaluated sozinibercept 2 mg in combination with aflibercept 2 mg for wet age-related macular degeneration
The study demonstrated that the number of injections needed gradually decreased during the follow-up period

Inherited retinal diseases have disease processes distinct from age-related macular degeneration and geographic atrophy, but may present with "phenocopy" clinical signs
Investigators measured the choroidal structures using enhanced depth imaging optical coherence tomography

SpliceBio continues to actively enroll patients in the ASTRA study and accompanying POLARIS trial

A clinician panel illuminates current treatment paradigms and best practices for reducing treatment burden
Additionally, the prognosis of macular oedema may help clinicians evaluate renal function, investigators reported

Clinicians react to the US approval of revakinagene taroretcel-lwey (Encelto; Neurotech Pharmaceuticals) for macular telangiectasia type 2

In a keynote speech, Warren Hoburg and Tyson J. Brunstetter, OD, PhD, will discuss in-orbit imaging and spaceflight-associated neuro-ocular syndrome

The encapsulated cell therapy from Neurotech Pharmaceuticals is the first FDA-approved treatment for MacTel

The modifier gene therapies from Ocugen target geographic atrophy and Stargardt disease
One in vitro study included in the manuscript was conducted in collaboration with researchers at the University of Southhampton in the United Kingdom

The investigators' findings suggest that CVI is a valuable noninvasive predictive biomarker for monitoring vascular changes in patients who smoke

The researchers reported a “notable” correlation which could serve as a predictive biomarker
The initiative marks the second annual ROP Awareness week, running from 24 February to 2 March, 2025
Researchers indicated that paediatric patients with Leber congenital amaurosis experienced improvements in vision

The biosimilar to aflibercept (Eylea) has already received approval in the EU and US

Francesco Bandello, MD, FEBO, discusses the Diabetic Eye Conditions Coalition and a five-point call to action
Risk factors vary in both short-term and long-term periods following Boston Type 1 keratoprosthesis surgery
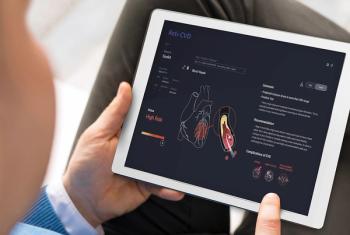
The UK-based company will debut the tool, called Dr.Noon CVD, at two conferences in March
Professor Anat Loewenstein, MD, speaks about her presentation from the 22nd Annual Angiogenesis, Exudation, and Degeneration Meeting

The authors evaluated shallow irregular pigment epithelial detachment, subretinal hyperreflective material, subretinal fluid, intraretinal fluid and other baseline OCT biomarkers

Researchers screened for outcomes such as the presence of retinal diseases including age-related macular degeneration and retinal vein occlusion
The implant was tested in eyes of patients with bilateral age-related macular degeneration who underwent cataract surgery
Choroidal and retinal changes were examined with optical coherence tomography angiography