
Classification of new EDOF lenses allows surgeons to make an informed choice for the patient undergoing cataract surgery, to improve their quality of life without spectacles.

Classification of new EDOF lenses allows surgeons to make an informed choice for the patient undergoing cataract surgery, to improve their quality of life without spectacles.

Novel trial design will compare safety and effectiveness outcomes for the OMNI Surgical System and the iStent inject in lowering IOP as a standalone treatment option without concomitant cataract surgery.

Cataract surgery in children is challenging because of the likelihood of posterior capsule opacification. This can be overcome with primary posterior laser capsulotomy coupled with bag-in-the-lens technology.

A very-high-frequency ultrasound device provides a range of detailed intraocular measurements that enable highly accurate ICL sizing and placement.

A team of investigators at the Okinawa Institute of Science and Technology Graduate University in Japan have identified a gene necessary for the survival of retinal ganglion cells—a class of neurons located in the retina that are critical for vision.

Investigators theorize that the ophthalmic nerve may be affected by the COVID-19 virus, and therefore may affect the corneal esthesiometry values.

The drug is prescribed as adjunctive therapy for those with glaucoma.

Investigators find that fusing human retinal cells with adult stem cells could be a potential therapeutic strategy to treat retinal damage and visual impairment.

Short-term steroid use can alleviate patient discomfort.

Researchers believe that the prolonged effect of bimatoprost may result from up-regulation of matrix metalloproteinases, leading to sustained remodelling of the outflow pathways.

Sub-retinal injection of adeno-associated virus leads to visual acuity gains.

Ultrasonic retinal prosthesis has been achieved by a research group at UCLA. It is a step towards a non-invasive retinal prosthesis that works without invasive eye surgery.

While investigators have found a link between dry eye and depression, the exact reasons behind the link are not immediately known.
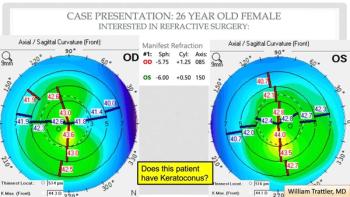
A team of investigators has found that the eye disease is more prevalent than first thought.

Investigators find that oestradiol drops can ease signs and symptoms for 3 months.

Glaukos Corp’s iLink therapy for the treatment of keratoconus consists of novel single-use drug formulations that are bio-activated by proprietary systems through the delivery of ultraviolet light to the cornea to induce corneal cross-linking designed to strengthen, stabilise and reshape the cornea.

Retinal changes seen in suspected transient ischaemic attacks are not helpful for predicting ischaemic events.

According to Dr Claudia Perez-Straziota, persistent epithelial defects do not have to live up to their name—they can be healed.
A team of investigators found that sigma 1 receptor, which is known to protect cells from stress, could turn out to be key to the function and survival of the neurons most impacted by glaucoma.

Russian bombs on Wednesday struck a hospital in Mariupol, Ukraine, damaging several buildings, including an ophthalmology department. Elsewhere, physicians are participating in relief efforts.

Many low- and middle-income countries lack resources for managing eye health. A pilot project aims to reduce the number of people going blind from untreated glaucoma by raising awareness and increasing engagement with screening and follow-up.

New World Medical notes that the intuitively designed Streamline features ClickPulse technology that targets the eye’s conventional outflow pathway.

Compounded medications are playing a central role in the process.
Recent innovations in glaucoma have helped clinicians and patients manage the condition more effectively.

The glaucoma toolbox is expanding to include laser, drug delivery systems and minimally invasive glaucoma surgery.

The companies note that the partnership will propel Ray Therapeutics’ lead optogenetics gene therapy to Phase 1-2 clinical trials.


The healthcare sector has high greenhouse gas emissions, to which ophthalmology is a significant contributor. Reducing waste, reusing instruments and medication and increasing our use of telemedicine can all contribute to reducing the carbon footprint of our specialty.
Utilizing new technology, surgeons can be 20 to 40 times more precise.

Researchers compared two novel lens designs using data obtained from 19 investigational sites in Australia, Canada, Spain and the UK.