
A novel computational platform identifies top-performing viral vectors that could deliver gene therapies to the retina with maximum efficiency and precision.

A novel computational platform identifies top-performing viral vectors that could deliver gene therapies to the retina with maximum efficiency and precision.

According to the Nanoscope Therapeutics, 6-month safety and efficacy data are expected in Q1 2023. MCO-010 gene therapy reprograms healthy retinal cells to make them photosensitive.
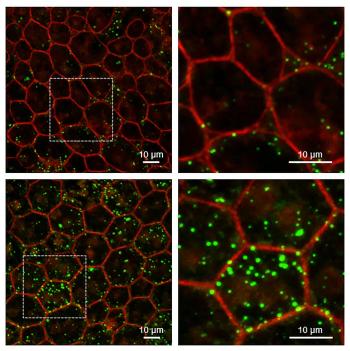
Eyes of mice lacking protective protein show signs similar to age-related macular degeneration.

RegeneRX Biopharmaceuticals and its US joint venture partner and licensee, HLB Therapeutics, signed a letter of intent this week with a global ophthalmology contract research organization to conduct a pair of Phase 3 clinical trials simultaneously beginning in the fall in the US and Europe for patients with neurotrophic keratitis.
Kiora Pharmaceuticals announced it has enrolled the first patient as a part of a Phase 2 study evaluating KIO-201 topical eye drops in patients with PCED, a rare ocular surface condition characterized by non-healing wounds on the eye surface.

Researchers at the University of Bristol, as part of a 10-year follow-up study, found that severe “brain bleeds” experienced by some babies in the first year following their birth lead to long-term sight problems.
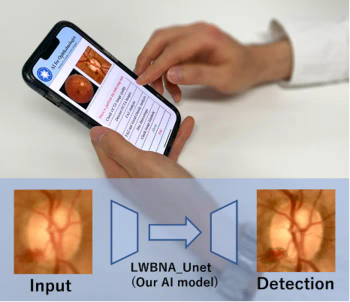
A new deep learning model that can identify disease-related features from images of eyes has been unveiled by a group of investigators from Tohoku University.

While the DAZZLE trial failed to meet its primary endpoint, KSI-301 demonstrated good initial visual gains and anatomic effects as well as positive durability.

A team of investigators from the Yong Loo Lin School of Medicine at the National University of Singapore has found that there is a bidirectional association between chronic kidney disease and glaucoma.

In a recent study, investigators found preclinical evidence that this innovative, biodegradable microneedle, dip-coated with a therapeutic drug for release upon insertion into the eyeball, can also be equipped with a special hydrogel that simultaneously seals off the insertion hole.
A team led by researchers from the University of Geneva has identified a molecular mechanism that causes degeneration of the eye’s photoreceptors, which can lead to blindness.

A team of investigators at Flinders University in Australia have found that a specific cell within the retina appears to be particularly good at housing Ebola and other viruses.

Via four cases, Dr Harry Flynn describes a variety of endophthalmitis management options for retinal surgeons.

According to investigators, a key reason for the link may lie in circadian “clocks,” the molecular machinery within every cell of every organism, which have evolved to adapt to daily stresses, such as changes in light and temperature caused by the rising and setting of the sun.

Dr Sally Tucker of Ora Europe walks through the clinical trial pathway, describing how Ora addresses complexities to make the path as efficient as possible.

Jeff Cleland, PhD, CEO of Ashvattha, discusses safety data for an at-home subcutaneous anti-VEGF injection option in development for the treatment of wet AMD and DMO.

The study assesses retinal blood biomarkers using a new prototype OCT, aiming to measure retinal biomarkers such as blood flow volume, average velocity and vessel diameter with a new prototype.

Dr Carlos Quezada Ruiz, senior medical director at Genentech, discusses “Predicting optimal treatment regimen for patients with neovascular age-related macular degeneration using machine learning.”

According to Novartis, the approval is based on year 1 data from the Phase III KESTREL and KITE clinical trials investigating brolucizumab-dbll 6 mg vs aflibercept 2 mg in diabetic macular oedema patients.

The study evaluates three separate cases, each of which experienced irreversible changes in their corneal structure caused by challenges in getting timely treatment due to various reasons, including insurance, lost to follow up, and the COVID-19 pandemic.

In a paper, investigators identified strategies used by patients to reduce the cost of therapy and its impact on adherence to treatment. Patients may be reluctant to disclose challenges regarding adherence to dry eye disease therapy, as well as fears of worsening quality of life.

An investigator at Anglia Ruskin University is leading work on an anti-cataract drug, which has had positive results in lab tests.
BRIM Biotechnology Inc. has entered into a new strategic partnership with Ora Inc. for the late-stage clinical development of lead drug candidate BRM421 for dry eye syndrome (DES).
In research sponsored by Skye Bioscience Inc., investigators at the University of Mississippi have demonstrated stronger and longer-lasting reduction of intraocular pressure when a proprietary molecule, SBI-100, is formulated as a nanoemulsion containing the mucoadhesive agent Carbopol 940.

Mike Watson, Vice President of OraNet, unpacks some of the most pressing challenges to clinical research in today’s climate.

Dr David Bingaman of Ora Clinical discusses today’s need to accelerate clinical studies and increase investments in retina and challenging disease states.

The trial marks the first-ever in vivo delivery of an experimental CRISPR gene editing medicine to a paediatric patient, with the company on track to complete dosing of the paediatric mid-dose cohort in the first half of 2022.

A team of investigators from Pohang University of Science and Technology has found that conjunctival goblet cell examination is important for the precise diagnosis and effective treatment of ocular surface diseases; however, CGC examination has not been possible until now due to lack of non-invasive devices.

Approval is based on year 1 data from the Phase III KESTREL and KITE trials investigating brolucizumab 6 mg versus aflibercept 2 mg in DMO patients.

The company’s announcement marks first clinical trial in humans of Ocugen’s modifier gene therapy platform.