
The pandemic reshapes treatment intervals for neovascular AMD, revealing flexible strategies that maintain visual outcomes and enhance patient care.

The pandemic reshapes treatment intervals for neovascular AMD, revealing flexible strategies that maintain visual outcomes and enhance patient care.

AI revolutionizes retinal care, enhancing diagnosis, monitoring, and treatment through innovative technologies and collaborative efforts in ophthalmology.

Research reveals how COVID-19 disrupted anti-VEGF treatment schedules, yet flexible regimens maintain visual outcomes for neovascular AMD patients.
Dr. Quan Dong Nguyen presents interim data from the phase 3 DRAGON study on a potential first therapy for adolescent Stargardt disease.

Explore the challenges and insights in long-term follow-up of paediatric gene therapy, focusing on data integrity, patient retention, and ethical considerations.

The EU-ROP registry revolutionizes retinopathy of prematurity management, offering insights into treatment variations and outcomes across Europe.


Dr. Hartnett highlights new approaches to ROP care and the changing ethical, clinical, and technological landscape in a guest lecture.

Dr. Sharon Fekrat presented a retrospective analysis assessing how antiplatelet and anticoagulant therapy relates to hemorrhage characteristics and outcomes.
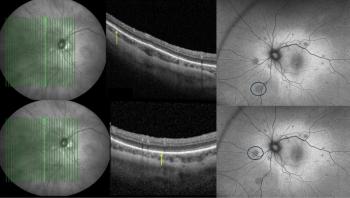
Priyanka Sanghi, BSc, MSc, MBBS, highlights how fundus autofluorescence and optical coherence tomography may help uncover long-term retinal footprints invisible to standard examination.

Prof Dr Rajvardhan Azad explains how unified guidelines, AI-driven tools, and cross-disciplinary collaboration could transform outcomes for premature infants.

Dr. J. Peter Campbell discusses the technologies shaping retinopathy of prematurity detection, clinical integration, and global implementation.

The two meetings will be held concurrently from December 4-7 in Florence, Italy


The newly-approved technology delivers low-dose microcurrents through closed eyelids to stimulate retinal cells

Leading companies spotlight innovations in cataract and refractive surgery at the 43rd ESCRS Congress in Copenhagen

At the ESCRS meeting, cataract surgeons can get familiar with BVI's Virtuoso platform and VirtuaLens' immersive VR IOL simulator

Alcon, Bausch + Lomb and Johnson & Johnson will debut new research findings during the European Society of Cataract and Refractive Surgeons Congress in Copenhagen, Denmark
Ahead of the ESCRS congress and iNovation day, we go behind the scenes with Ciliatech founder Philippe Sourdille, MD, and president Olivier Benoit

Presentation topics will include therapies for age-related macular degeneration, X-linked retinitis pigmentosa and other retinal pathologies

Clinical leaders including Susan Schneider, MD; Lejla Vajzovic, MD; and Harvey Uy, MD, will share new clinical findings

The Global RDH12 Alliance unites inherited retinal disease advocacy groups including UK-based Eyes on the Future and the US-based RDH12 Fund for Sight

Exonate initiates a phase 2b trial for EXN407, a groundbreaking eye drop treatment for diabetic retinopathy

Researchers at the Korea Advanced Institute of Science and Technology demonstrated the next-generation strategy for sustainable synthesis


A precautionary recall of Zaditen eye drops is issued due to potential microbial contamination, affecting batch 4V64. No adverse events reported.

ZTech-L and ZTech-P, as part of the ZTech portfolio, supports administering therapeutics in both liquid and dry powder formulations

Three dose level cohorts of the small interfering RNA therapeutic were evaluated in a double-blind study

Teprotumumab is the first therapy specifically licensed for the treatment of moderate-to-severe thyroid eye disease in the UK

Utilizing artificial intelligence (AI), Cascader is a new medical technology company that aims to transform the detection and management of eye disease.

Published: December 6th 2025 | Updated:

Published: May 8th 2025 | Updated:

Published: April 11th 2025 | Updated:

Published: December 13th 2025 | Updated:

Published: December 7th 2025 | Updated:

Published: June 5th 2022 | Updated: