
Recent research suggests the need for routine HSV-1 and VZV PCR testing in all explanted corneas regardless of clinical suspicion to detect, treat, and prevent possible recurrence of herpes infection in corneal grafts and support graft survival.

Recent research suggests the need for routine HSV-1 and VZV PCR testing in all explanted corneas regardless of clinical suspicion to detect, treat, and prevent possible recurrence of herpes infection in corneal grafts and support graft survival.
A recent survey of retinal clinic patients reveals a lack of awareness of the risks and safety issues associated with stem cell therapies.

Inflammation is par for the course in ocular gene therapy; preventing it should be the goal because once it develops, treatment can be difficult.
An end-of-week review of what happened in ophthalmology from 29 October to 3 November 2022.
Clinicians need to investigate patients’ history, family history, visual behaviours and general health in order to promptly and accurately refer paediatric cases to ocular genetics clinics.

Companies are exploring light-sensitive proteins method in patients with advanced disease.

According to one study, when genetic testing is coupled with advanced imaging, the chances of identifying keratoconus early are greater.

A synopsis of the findings presented at EURETINA 2022 for ophthalmologists and retina specialists.

Foundation Fighting Blindness is a driving force in advancing retinal gene therapies into clinical trials.

Recent reports of retinal atrophy have raised concerns on potential long-term safety.
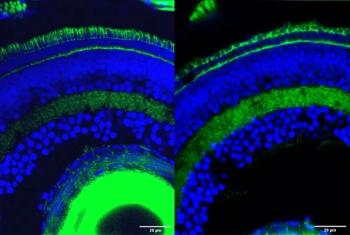
The findings of this study may have revealed the crucial role of gene fragments in retinal development and vision.

A novel computational platform identifies top-performing viral vectors that could deliver gene therapies to the retina with maximum efficiency and precision.

According to the Nanoscope Therapeutics, 6-month safety and efficacy data are expected in Q1 2023. MCO-010 gene therapy reprograms healthy retinal cells to make them photosensitive.

Metformin may provide AMD protection; DAVIO presents positive 12-month results; TOWER study reveals new imaging index for AMD.

Topics spanned from faricimab in nAMD and DME; pediatric retinal detachment surgery; and the relationship between outer retinal integrity and subretinal fluid.

Increasing understanding of the genetics behind Usher syndrome suggests that treatment may become possible in the very near future.

Promising gene therapies in development are pinpointing steps in the pathogenic cascades involved in ocular diseases, such as VEGF for wet AMD and CFI for dry AMD.

Options under investigation for the treatment of AMD include interruption of the dry AMD disease process and gene therapy to prompt the retina to heal itself.

An end-of-week review of what happened in ophthalmology from April 9-April 15.

The company’s announcement marks first clinical trial in humans of Ocugen’s modifier gene therapy platform.

Sub-retinal injection of adeno-associated virus leads to visual acuity gains.
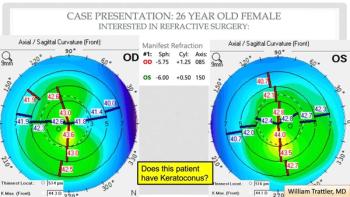
A team of investigators has found that the eye disease is more prevalent than first thought.

The companies note that the partnership will propel Ray Therapeutics’ lead optogenetics gene therapy to Phase 1-2 clinical trials.

A brief overview of findings presented at the 2022 Angiogenesis, Exudation and Degeneration meeting hosted by Bascom Palmer.
Investigators in the Netherlands have developed an RNA therapy to halt the progression of Stargardt disease.