
The investigators evaluated corrective surgery procedures to determine their effect on ocular outcomes.

The investigators evaluated corrective surgery procedures to determine their effect on ocular outcomes.

The company seeks to replicate European success of the ELIOS procedure in the US market.

The pharmaceutical company will present study results at the World Glaucoma Congress.

Horizon Therapeutics announced the Brazilian Health Regulatory Agency (ANVISA) has approved TEPEZZA as the first and only medicine approved in Brazil for treatment of thyroid eye disease.

The company announced the Complete Response Letter from the FDA for the Biologics License Application for aflibercept 8 mg is “solely due to an ongoing review of inspection findings at a third-party filler.”

The live streamed event will cover topics including DME, CME, AON and more.

According to researchers, the new imaging approach reveals changes in retinal microcirculation may indicate brain diseases that involve reduced blood flow.
The two winners were awarded a €60,000 prize for their work to treat degenerative retinal disease.

Researchers totaled the societal and healthcare costs of inherited retinal diseases.

AG-80208 is a novel, first-in-class, formyl peptide receptor (FPR) agonist formulated as an aqueous solution eye drop to treat the inflammation linked with DED.

The lenses are designed to correct and prevent myopia in children ages 6 to 14.
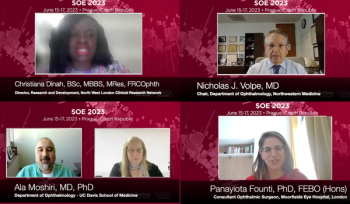
Did you miss the 2023 European Society of Ophthalmology congress? Don't worry – you can catch up with our video coverage.
Antimicrobial resistance is resistance to multiple classes of antibiotics, which makes it extremely challenging to treat infections caused by such organisms.
Artificial intelligence (AI) is going to be highly instrumental in patient care in all medical specialties and will be highly relevant in eye care.
Dr Ala Moshiri provides an overview of current treatment options for diabetic macular oedema.
Jorge L. Alió, MD, PhD, FEBOphth, shared his pearls situations in which a patient presents with a cataract and corneal opacity at the European Society of Ophthalmology Conference, Prague.

Paracentral acute middle maculopathy can develop after excessive intake of caffeine and alcohol according to Obadah Moushmoush, MD.
Omnigen® may be an alternative treatment option for patients with a persistent corneal epithelial defect. The product provides a convenient and effective treatment that can be administered in an outpatient setting.

As more countries receive approval for the use of faricimab, patients are going to continue to have good visual outcomes

Scientists hope to develop novel AI solutions for the prediction of DPN using corneal OCT images.

The new company debuted with £96 million in funding to target retinal diseases.

The EACCME accreditation is renewed through 2025.

The trial is evaluating Oxurion’s novel plasma kallikrein (PKal) inhibitor THR-149 as a potential treatment for DME patients who respond suboptimally to anti-VEGF therapy.
RAFARM and BioNanoSim have entered into an agreement to create the company.

A look at how EDOF technology can be categorised into five types

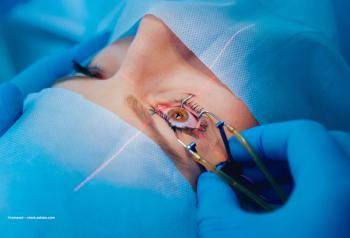
Debate continues over the best way to apply treatment option

SOE holds a special place in its heart for the up-and-coming young ophthalmologists and is providing them a number of opportunities to hone their skills and establish professional contacts at this meeting.

Novel procedures may hold greater appeal for cataract and refractive surgeons

The European Society of Ophthalmology (SOE) 2023 Congress will convene from June 15 to 17 in Prague, Czech Republic.