
At ASCRS2024, George O. Waring IV, MD, FACS, provides an overview of his presentation highlighting outcomes for LASIK performed using an updated laser ablation algorithm

Hattie Hayes is the editor of Ophthalmology Times Europe.

At ASCRS2024, George O. Waring IV, MD, FACS, provides an overview of his presentation highlighting outcomes for LASIK performed using an updated laser ablation algorithm

Filomena Ribeiro, MD, PhD, FEBO, discusses her presentation on neuroadaptation following the ASCRS Refractive Day

Sean Hanlon, CEO of CoFi, details the patient and provider benefits of recent CareCredit integration

Rich Small, CEO of Neurotech Pharmaceuticals, provides an update on the months ahead for NT-501 encapsulated cell therapy

Zeiss has secured regulatory approvals to acquire DORC and will shift focus to integration implementation

The glaucoma specialist talks about navigating the world of research, and the realities of motherhood, as a clinician

The opportunities and challenges presented by CRISPR manipulation

The European Commission is expected to make a decision about the application before the end of June

Retina specialists Giuseppe Querques, MD, PhD, and Paolo Lanzetta, MD, debate at the Congress on Controversies in Ophthalmology

At this year's Congress on Controversies in Ophthalmology, keynote speaker Ursula Schmidt-Erfurth, MD, addressed the clinical use of artificial intelligence

The retina specialist shares advice for the clinician-scientists of a digital-centric future

Filomena Ribeiro, MD, PhD, FEBO, discusses the crucial role of mentors – and where to find them

The approval marks the first new steroid on the US ophthalmic market in more than 15 years

The algorithms could provide major accessibility improvements and cost savings to underdeveloped regions

The company made its CE Mark announcement during the ESCRS Winter Meeting
The technology has potential for applications in contact lenses and intraocular implants

An investment from the Sir Jules Thorn Charitable Trust will support a state-of-the-art building, opening in 2027

Dr Jennifer Lim reveals new data on Angiopoietin-2 inhibition and insights on clinical trial design

Anat Loewenstein, MD, illuminates the outcomes of using an AI-based fluid quantification for remote retina monitoring and analysis

Dr Carl Regillo provided an overview of topline results – and a deep dive into subgroup analyses – of DAVIO 2 trial data

An in-depth case study conversation

Euan Thomson, Head of the Ophthalmology Strategic Business Unit and Head of the Digital Business Unit at Carl Zeiss Meditec, discusses plans for the company's vitreoretinal surgery workflows following announcement of DORC acquisition

The European Union approval applies to aflibercept 8 mg for treatment of nAMD and DME

A review of our most important content from throughout the year

Experts from around the globe converged in San Francisco, California

In early November, the EU MDR issued a CE mark certification for RetInSight’s AI-based geographic atrophy monitor

At this year's ESCRS meeting, industry leaders shared advice for young ophthalmologists–and what they wish they'd known earlier in their careers
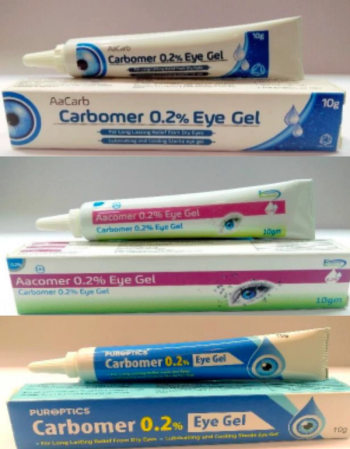
The carbomer-containing eye gels carry risk of microbial contamination, the regulatory agency warned

Dr Marco Zarbin emphasises the importance of defining “active disease”

Mark Blecher, MD, spoke with the Ophthalmology Times team about his poster addressing retinal visualisation and patients who have undergone small aperture IOL implantation at this year's American Academy of Ophthalmology meeting