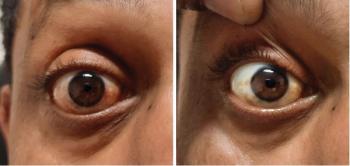
European patients navigate new therapies and market change-ups

European patients navigate new therapies and market change-ups

The drop breaks up degraded protein complexes which cause lens clouding

Specular microscopy showed "long-term alterations" in corneal cells

Smaller surgeries, in greater numbers, await new glaucoma specialists

Determining the medication’s safety and efficacy for paediatric patients

Lupin has successfully completed a clinical study of LUBT010, a ranibizumab biosimilar to Lucentis

The biosimilar is indicated to improve and maintain visual acuity for patients with neovascular age-related macular degeneration

Diabetic Retinopathy ranked as the fifth most common cause of blindness among adults aged 50 and older in 2020 according to data from the Global Burden of Disease study. Aude Couturier, MD shares her experience of using advanced imaging technologies to optimize diagnosis and surgical management of her DR patients in our latest Case of the Month.

Stabilising keratoconus and improving vision using ELZA-PACE customised CXL

Identifying all genetic culprits may improve diagnostics and prognoses

A postcard from the American Society of Retina Specialists meeting in Stockholm

The ALLY Adaptive Cataract Treatment System will be the first EU ALLY commercial installation


A list of 14 risk factors indicate dementia is not an inevitable consequence of aging

The nonprofit Prevent Blindness recognised the awareness month with a slate of resources
In a press release, Roche noted that up to 60% of patients were able to extend treatment intervals to 3 or 4 months

The EMA issued a designation for the small molecule photoswitch, which targets inherited retinal diseases including retinitis pigmentosa

Femtosecond laser, image-guided, high-precision trabeculotomy (FLigHT) received approval for patients in Europe with primary open-angle glaucoma

The positive opinion comes with the recommendation that ciclosporin 0.1% be granted marketing authorisation in the European Union

Companies in the retina space announced presentations ahead of the American Society of Retina Specialists meeting, to be held 17-20 July

Investigators conducted a case-control study to investigate the corneal topography parameters and biomechanics in patients with alopecia areata

The organisation has secured £72.75 million for its new eye health centre

A novel formulation may prevent blood vessel growth and vascular leakage in the retina
GLP-1 receptor agonists, such as Wegovy, Ozempic and Novo Nordisk, are becoming more prolific

The company has secured $170 million (approximately €157 million) in Series B funding

Extended daylight hours and plentiful outdoor space make Stockholm a memorable destination for meeting attendees
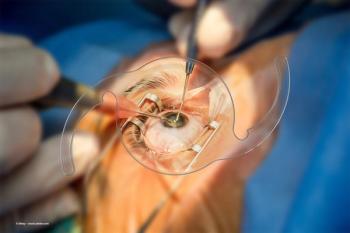
Patients with corneal astigmatism underwent phacoemulsification and rotationally asymmetric multifocal IOL implantation

Educational resources include handouts, videos and a dedicated webpage on the proper way to use eye drops

Following the MAA in Europe, Altos Biologics plans to pursue approval in target markets including Korea
Faricimab is already approved in the EU and UK for neovascular age-related macular degeneration and diabetic macular oedema