Retina
Latest News
Video Series

Latest Videos
Podcasts
CME Content
More News


The EU-ROP registry revolutionizes retinopathy of prematurity management, offering insights into treatment variations and outcomes across Europe.


Dr. Hartnett highlights new approaches to ROP care and the changing ethical, clinical, and technological landscape in a guest lecture.
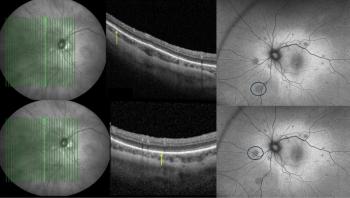
Priyanka Sanghi, BSc, MSc, MBBS, highlights how fundus autofluorescence and optical coherence tomography may help uncover long-term retinal footprints invisible to standard examination.

Prof Dr Rajvardhan Azad explains how unified guidelines, AI-driven tools, and cross-disciplinary collaboration could transform outcomes for premature infants.

Dr. J. Peter Campbell discusses the technologies shaping retinopathy of prematurity detection, clinical integration, and global implementation.

Kiora Pharmaceuticals advances ocular therapies with new patents for KIO-104, targeting retinal inflammation and enhancing treatment options.

The company noted that this approval marks Celltrion's first Health Canada-approved biologic product in ophthalmology.

The EXTEND trial provided 5-year follow-up for 10 patients with advanced retinitis pigmentosa, who had previously received MCO-010 during a phase 1/2a trial

SPVN20 is a gene-agnostic, intravitreal AAV-based gene therapy that targets dormant cones to restore vision, according to SparingVision

The satellite of the US-based University of Miami Health System will offer a “full spectrum of ophthalmic services” in the United Arab Emirates

Reporting from the European Society of Retina Specialists annual congress

The investigators noted that this report is the first about subretinal drusenoid deposits in Black and Hispanic patients with age-related macular degeneration
Patients with proliferative diabetic retinopathy, central retinal vein occlusion and branch retinal vein occlusion experienced fluctuating retinal perfusion, investigators reported

Biomarkers and fine-tuned staging are key to progress in improving retinal care, according to Fanka Gilevska, MD, PhD


Patient response focused on perceived vision-related quality of life outcomes, investigators said

EyeArt uses artificial intelligence for autonomous detection of vision-threatening diabetic retinopathy

The current staging system fails to fully capture the neurovascular unit's status, said Stela Vujosevic, Md, PhD, FEBO

At the EURETINA congress, Prof Boon discussed controversies in treatment, some driven by critical prospective studies

Alteogen received a positive CHMP opinion for EYLUXVI in July 2025


Artificial intelligence is quickly expanding the breadth and depth of information clinicians can interpret, Prof Uy said

Prof Stanzel detailed new advancements in proliferative vitreoretinopathy and a hyperaspheric IOL designed for patients with age-related macular degeneration