Onboarding a new trifocal hydrophobic C-loop IOL My early experiences with AT ELANA® 841P from ZEISS
Good vision at near and intermediate distances is important in today’s world where so much time is spent using smartphones and computers. For patients who are interested in achieving spectacle independence after cataract surgery, IOL manufacturers have sought to address this need by introducing a variety of presbyopia-correcting IOLs based on different optical designs to provide an extended range of vision. The hydrophobic acrylic ZEISS AT ELANA 841P, a new trifocal fully preloaded IOL (Carl Zeiss Meditec, AG; Jena, Germany), became one of the latest entries into the category of presbyopia-correcting IOLs.
The AT ELANA 841P combines leading technologies when it comes to optical performance, biomaterial properties, and IOL design. Namely, the AT ELANA 841P optic design is based on that of the trifocal AT LISA® tri 839MP. There is abundant clinical proof that the AT LISA tri delivers good uncorrected vision for near and intermediate without compromising distance visual acuity.1,2 Distinguishing itself from the AT LISA tri, the AT ELANA optic was modified to have increased light transmission efficiency and a higher allocation of light towards near vision to enhance near-to-intermediate vision without compromising far vision.* In addition, AT ELANA 841P features a spherical aberration neutral design and stays fully trifocal regardless of pupil size.
The biomaterial and platform design of the AT ELANA 841P are features shared with the monofocal ZEISS CT LUCIA 621P IOL. The AT ELANA 841P is made of a glistening-free** hydrophobic acrylic biomaterial with a heparin-coated*** surface to ensure optical clarity and smooth and controlled unfolding of the IOL.
Figure 1. ZEISS AT ELANA 841P features the best of ZEISS trifocal technology on a hydrophobic C-loop platform.

Getting started with the AT ELANA 841P
I took notice of the AT ELANA 841P because it has features that I prioritize when choosing a presbyopia-correcting implant for my cataract surgery patients. Specifically, if the patient meets certain parameters, I prefer diffractive trifocal technology because I believe it gives patients the best chance of obtaining good quality vision at near, intermediate, and distance. In addition, I favor hydrophobic lenses because the development of posterior capsule opacification (PCO) tends to be slower, and anterior capsule contraction has also been shown to be lower with the hydrophobic material.3,4 Further reducing the chance of PCO, the AT ELANA 841P optic has a 360° sharp edge design. I also appreciated that the AT ELANA’s biomaterial reduces the risk of glistening and that the IOL comes fully preloaded in the easy-to-use BLUESERT™ injector; preloading minimizes the risk of IOL damage and increases surgical efficiency and safety. Finally, the AT ELANA 841P is available in a wide dioptric range (0.0 to +34.0 D).
Still, it is my practice to proceed cautiously when trying any new IOL, even more so with premium IOL technologies. I begin by reviewing findings from bench testing to see if the results for modulation transfer function, light distribution for different foci, and point spread function support claims about range of vision, vision quality, and visual symptoms. If the laboratory evidence is convincing, I begin using the new lens in a limited number of patients who have the characteristics that I consider ideal for the IOL based on its optical bench performance. Trialing the lens in this way allows me to see if the achieved results match my expectations. I also like to review my early refractive outcomes to determine the need for lens constant optimization.
Patient selection criteria and early outcomes
I was satisfied by the in vitro test results for the AT ELANA 841P showing it had good optical performance across an extended range of distances that was at least comparable to the AT LISA tri and slightly better at near to intermediate distances.5 The patients I considered as candidates for my initial use of the AT ELANA 841P were identified by their interest in achieving true spectacle independence after cataract surgery. All patients received thorough preoperative counseling to explain potential pros and cons and set realistic expectations. I usually do not implant a multifocal IOL in any patient whose occupation involves night driving because the potential for halos and glare is a limitation associated with all multifocal technology.5 I also do not offer multifocal IOLs to any patient with severe dry eye because a stable tear film and healthy ocular surface are essential for achieving good quality vision with multifocal technology.
Because the AT ELANA 841P is not yet available in a toric version and taking into account my surgically-induced astigmatism, I excluded patients with astigmatism >1.50 D with-the-rule or >1.0 D against-the-rule. Considering that the AT ELANA 841P has an overall diameter of 13.0 mm and due to concern about the potential for decentration when implanting a lens with a diameter that exceeds the white-to-white (WTW) measurement, I also excluded patients with a WTW >12.5 mm. For the latter patients, I have an option for implanting a ZEISS trifocal IOL using the AT LISA tri instead.
I planned the AT ELANA 841P surgeries using the IOLMaster® 700 (Carl Zeiss Meditec, AG; Jena, Germany) for biometry and the ESCRS Calculator using the Kane formula for IOL power calculation with a target refraction of emmetropia. Surgery was performed through a 2.2 mm incision at the steepest meridian, and the AT ELANA 841P was delivered with a docking technique through the same incision. As exceptions to this approach, I operate through a 2.4 mm limbal incision in cases where the IOL power is >25-26 D.
Preoperatively, mean sphere for the six eyes of the first three patients I implanted was 1.10 D ± 2.12 D and mean cylinder -0.21 D ± 0.32 D. All patients returned for follow-up after 1 week, 1 month, and 3 months. Slit-lamp examination showed that all AT ELANA 841P IOLs were stable and well centered. Mean sphere was 0.033 D ± 0.25 D, and mean cylinder was -0.00 D ± 0.22D. Visual acuity (in decimal) results were excellent. Mean monocular UCVA was 0.96 ± 0.08, mean binocular UCVA was 1.0 ± 0, mean monocular DCVA was 1.0 ± 0, and mean binocular DCVA was 1.2 ± 0.
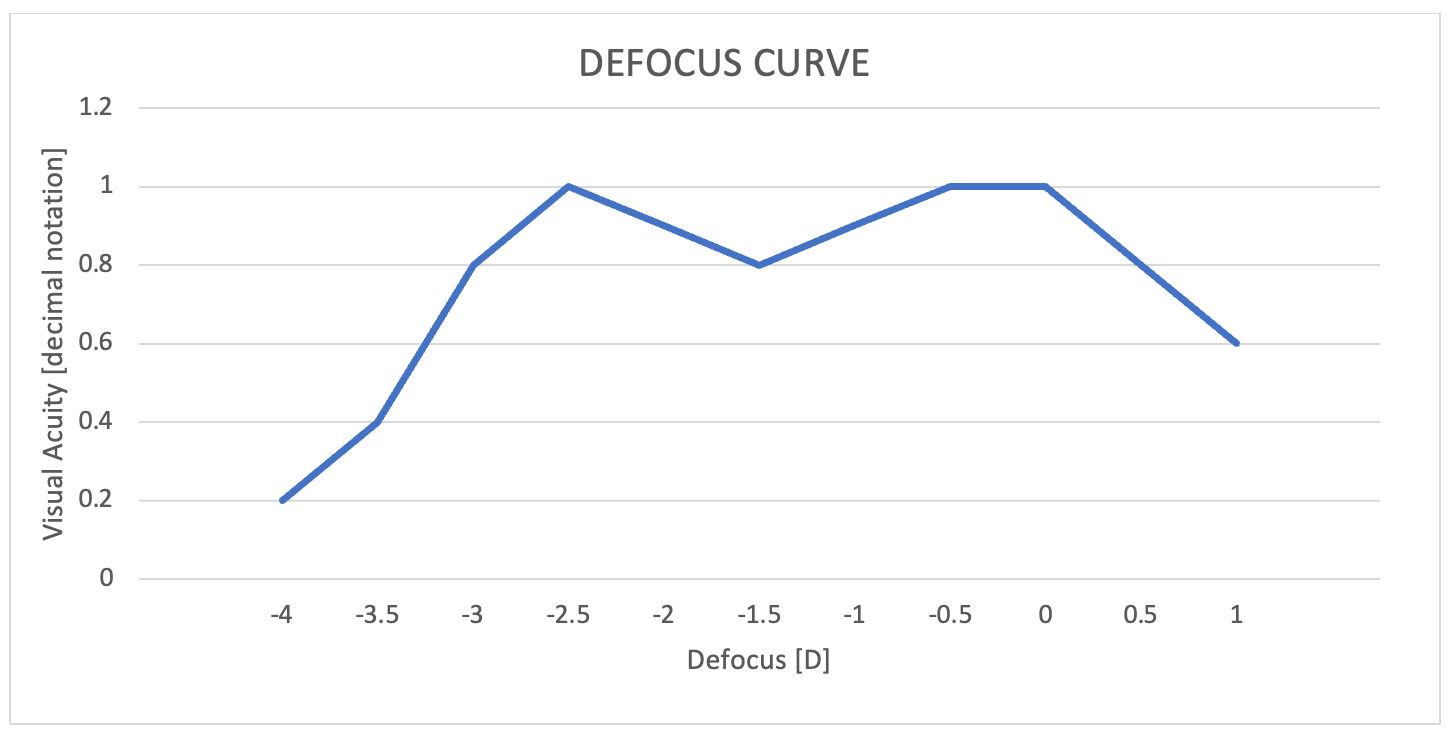
Patients were very happy with their outcomes and reported not needing glasses for daily activities despite being left slightly hyperopic, which suggests the AT ELANA 841P may be tolerant to minor refractive error. As shown by the binocular defocus curve for one patient, bilateral implantation of AT ELANA 841P IOLs provided a full range of good vision with VA (in decimal) of 1.0 at distance, 0.8 at intermediate (66 cm), 1.0 at near (40 cm) (Figure 2).
Figure 2. Binocular defocus curve from my first patient shows good visual acuity (decimal 0.8 to 1.0) is achieved from far through intermediate to near distances.
Two patients admitted to seeing halos when asked directly about specific visual symptoms, but no patient complained spontaneously about photic phenomena or said they were bothered by halos or glare.
Concluding thoughts
With its diffractive trifocal optic and hydrophobic acrylic material, the AT ELANA 841P matches two of my preferences for choosing a presbyopia-correcting IOL. Importantly, I found that in my early surgical experience, bilateral implantation of this new trifocal lens resulted in very satisfied patients who were happy with their ability to see well without correction at near, intermediate, and distance.
I will continue to review my outcomes with the AT ELANA 841P as my experience with it increases, and I expect I might even see some improvement because I optimized my lens constant. Currently, I feel comfortable both expanding use of the AT ELANA 841P in my practice and encouraging other surgeons to consider it.
* Compared to AT LISA tri in photopic conditions in virtual implantations and optical bench tests.
** Grade 1 (traces) or better for 85% of the patients up to and including 12 months according to Christiansen scale and based on internal clinical trial outcomes and on published clinical data.
*** Fragment of heparin used in IOL surface coating with no pharmacological, immunological or metabolic action.
References
- Mojžiš P, Majerova K, Plaza-Puche AB, Hrckova L, Alio JL. Visual outcomes of a new toric trifocal diffractive intraocular lens. J Cataract Refract Surg. 2015;41(12):2695-706.
- Mojžiš P, Kukuckova L, Majerova K, Ziak P, Piñero DP. Postoperative visual performance with a bifocal and trifocal diffractive intraocular lens during a 1-year follow-up. Int J Ophthalmol. 2017;10(10):1528-1533.
- Zhao Y, Yang K, Li J, Huang Y, Zhu S. Comparison of hydrophobic and hydrophilic intraocular lens in preventing posterior capsule opacification after cataract surgery: An updated meta-analysis. Medicine (Baltimore). 2017;96(44):e8301.
- Chen L, Zhong Y, Yao K, Fu Q. Effect of intraocular lens material and haptic design on anterior capsule contraction after cataract surgery: A systematic review and meta-analysis. Graefes Arch Clin Exp Ophthalmol. 2023 Oct 13. Epub ahead of print.
- Łabuz G, Yan W, Khoramnia R, Auffarth GU. Optical-quality analysis and defocus-curve simulations of a novel hydrophobic trifocal intraocular lens. Clin Ophthalmol. 2023;17:3915-3923.

Dr Luis Fernández-Vega Cueto-Felgueroso is Associate Professor of Ophthalmology, University of Oviedo, andConsultant and Surgeon for the Cornea, Cataract and Refractive Surgery Unit at the Instituto Oftalmológico Fernández-Vega in Oviedo, Spain.
