
Dr Baruch Kuppermann discusses interim results from the first in-human Phase 2 RIPPLE-1 Trial of a dexamethasone implant for diabetic macular oedema and retinal vein occlusion.

Dr Baruch Kuppermann discusses interim results from the first in-human Phase 2 RIPPLE-1 Trial of a dexamethasone implant for diabetic macular oedema and retinal vein occlusion.

Robyn Guymer, MBBS, PhD, reported the Voyager study’s preliminary data at the 2024 Angiogenesis, Exudation, and Degeneration conference

EODM may be an ideal candidate for development of complement gene therapy due to the high-impact genetic variants
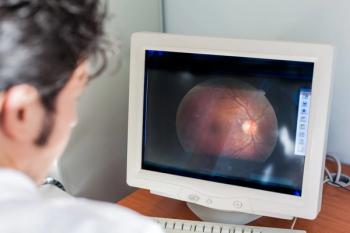
Researchers aimed to develop a translational map to understand how advanced imaging biomarkers manifest in typical diagnostic imaging

The afternoon sessions begin after lunch and continue well into the evening at the Angiogenesis, Exudation, and Degeneration 2024 meeting

The one-day event will be held online Saturday, February 3, 2024, and is sponsored by the Bascom Palmer Eye Institute, University of Miami Health System

A study conducted by Gemmy Cheung, MBBS, FRCOphth and colleagues shows geographic atrophy (GA) lesion phenotypes, associated features, and growth rates differ between Asians and non-Asians.

A brief overview of findings presented at the 2022 Angiogenesis, Exudation and Degeneration meeting hosted by Bascom Palmer.
Investigators in the Netherlands have developed an RNA therapy to halt the progression of Stargardt disease.
KALAHARI study finds THR-149 to be safe, well-tolerated with preliminary efficacy as treatment for DMO patients who respond suboptimally to anti-VEGF.
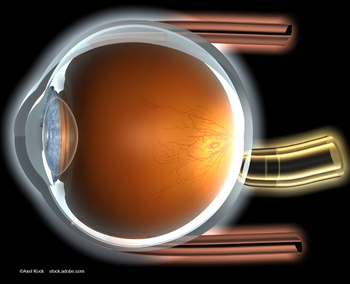
Most patients who had received monthly injections, that is, 92%, preferred the PDS after switching, with 88% having a very strong preference.
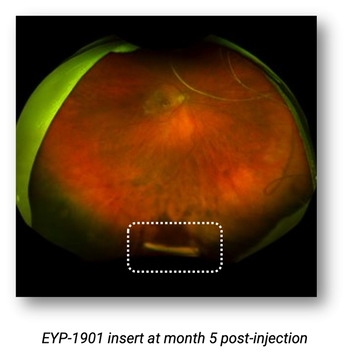
Previously treated patients showed significantly reduced treatment burden.

In a presentation at the Bascom Palmer Eye Institute’s 19th annual Angiogenesis, Exudation and Degeneration 2022 Virtual Edition, Dr Glenn J. Jaffe noted that the analysis showed, for the first time, a decreased growth rate in the central foveal area by a therapeutic intervention when compared with sham treatment.

This study showed that an investigational subretinal implant, CPCB-RPE1, containing allogeneic human embryonic stem cell-derived RPE cells, was safe and well-tolerated by patients with dry AMD.

A once-daily oral drug targets inflammatory processes and thus far has been found to be well tolerated in a Phase 2 safety trial.

Ophthalmologists gather to discuss neovascular and exudative diseases of the eye and the revolutionary therapies on the horizon.